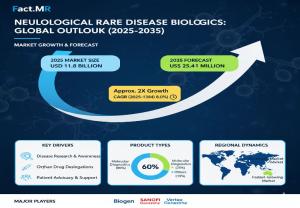
Neuro Biologics Market Valued at $11.8B in 2025, to Reach USD 25.4 billion by 2035
Neurological rare disease biologics market is projected to grow from USD 11.8 billion in 2025 to USD 25.4 billion by 2035, advancing at a CAGR of 8.0%.
ROCKVILLE, MD, UNITED STATES, October 15, 2025 /EINPresswire.com/ -- The neurological rare disease biologics industry stands at the threshold of a decade-long expansion trajectory that promises to reshape therapeutic infrastructure and advanced biopharmaceutical treatment systems. The market’s journey from USD 11.8 billion in 2025 to USD 25.4 billion by 2035, growing at a CAGR of 8.0%, underscores the accelerating adoption of advanced monoclonal antibody platforms, gene therapy innovations, and RNA-based biologics across neurodegenerative, neuromuscular, and neurodevelopmental disorders. This transformative growth not only reflects scientific innovation but also a broader shift toward precision therapeutics designed to modify — rather than merely manage — the course of rare neurological diseases.The landscape of neurological rare disease therapeutics is undergoing a fundamental transformation. Where once treatment options were limited to symptomatic care, a new generation of biologics — from antisense oligonucleotides and gene therapies to engineered antibodies and enzyme replacements — is tackling root causes. This shift is creating a specialized market that blends high scientific complexity with strong unmet clinical need, attractive regulatory incentives, and intense commercial interest. The result is a dynamic, high-value segment of biopharma where scientific breakthroughs translate rapidly into strategic pipelines and commercial partnerships.
Why This Market Matters Now:
Rare neurological diseases, by definition individually uncommon but collectively substantial, produce enormous personal and societal burden. Advances in molecular genetics have clarified disease mechanisms and identified actionable targets, enabling biologic modalities that can modulate gene expression, replace missing proteins, clear toxic aggregates, or re-engineer cellular function. Coupled with orphan drug incentives, adaptive regulatory pathways, and improved clinical trial designs for small populations, the environment now favors rapid translation. For investors, payors, and clinicians alike, neurological rare disease biologics represent both therapeutic promise and meaningful market opportunity.
Key Market Drivers:
Several forces are converging to propel the market. First, progress in discovery tools — human genetics, disease modeling with patient-derived cells, and sophisticated biomarkers — is enabling more tractable target selection. Second, delivery technologies (viral vectors, lipid nanoparticles, intrathecal administration techniques) are improving CNS access, a historical bottleneck for brain and spinal cord disorders. Third, regulatory frameworks and reimbursement pathways for rare diseases make economically challenging but clinically transformative products feasible. Finally, rising patient advocacy and registries are accelerating trial recruitment and natural history understanding, shortening development timelines.
Therapeutic Modalities Shaping Uptake:
The market is defined less by a single modality than by a bouquet of biologic approaches matched to disease biology. Antisense oligonucleotides (ASOs) and RNA-targeting platforms have delivered high-profile proofs of concept by altering splicing or knocking down pathogenic transcripts. Gene therapies — both viral vector–based gene replacement and gene editing approaches — are moving into the clinic for monogenic neurological disorders. Monoclonal antibodies and engineered proteins address extracellular targets and neuroinflammation, while enzyme replacement and substrate reduction strategies target metabolic and lysosomal diseases with neurological involvement.
Recent Developments and Innovation Signals:
Across the industry, several trends stand out. Licensing deals and strategic partnerships between platform owners and large pharma are commonplace, reflecting the need to combine discovery agility with global development and manufacturing scale. Advances in vector engineering, manufacturing scale-up, and standardized potency assays are lowering technical and commercial risk. Meanwhile, the field is experimenting with novel clinical endpoints, patient-reported outcomes, and digital biomarkers that better capture functional benefit in small cohorts. There is also growing interest in repeat-dosing strategies and approaches that enable reversible or tunable effects — important considerations in neurological indications where safety and long-term effects are paramount.
Regional Insights — United States and Europe:
The United States remains the epicenter for R&D activity and capital. A high concentration of biotech innovators, deep venture and corporate investment, and a regulatory environment that has demonstrated flexibility for high-unmet-need treatments combine to accelerate development. Many pivotal trials and early approvals occur first in the U.S., establishing precedents for global rollout.
Europe offers complementary strengths: world-class clinical research networks, strong public payor systems that prioritize demonstrable value, and regulatory incentives for orphan products. European centers of excellence often contribute critical natural history data and run landmark clinical programs. While market access negotiations in Europe can be more complex due to national reimbursement processes, successful launches there confer strong credibility and reach.
Key Players and Competitive Dynamics:
The competitive landscape mixes large, diversified pharmaceutical companies with specialist biotech innovators and academic spin-outs. Large players bring regulatory experience, manufacturing capacity, and commercial reach; smaller specialists contribute platform technologies and disease-centric expertise. Frequent partnerships, milestone-based licensing, and multi-asset collaborations are the rule rather than the exception. Companies that can demonstrate both rigorous safety data and durable clinical benefit will command premium positioning.
Request For Discount : https://www.factmr.com/connectus/sample?flag=S&rep_id=11201
Challenges and Barriers:
Despite the promise, the neurological rare disease biologics market faces meaningful hurdles. Achieving safe and effective CNS delivery remains technically challenging for many modalities. Manufacturing complexity and cost — especially for viral vectors and cell therapies — create economic pressure on pricing and reimbursement. Long-term safety, durability of response, and real-world evidence generation are essential but resource-intensive.
Buy Now This Report at USD(4500) :https://www.factmr.com/checkout/11201
Outlook and Strategic Takeaways:
The next decade will likely see a steady stream of biologic firsts across neurological rare diseases: incremental gains in ASO design, broader application of gene replacement and editing, and refined combination strategies for complex pathologies. Commercial success will favor organizations that pair cutting-edge science with scalable manufacturing, clear regulatory strategy, and engagement with payors to demonstrate long-term value.
Checkout Our Related Published Report :
Rare Neurological Disease Treatment Market: https://www.factmr.com/report/4594/rare-neurological-disease-treatment-market
Rare Musculoskeletal Disorder Treatments Market: https://www.factmr.com/report/rare-musculoskeletal-disorder-treatments-market
Rare Hemophilia Factors Market: https://www.factmr.com/report/rare-hemophilia-factors-market
About Fact.MR:
We are a trusted research partner of 80% of fortune 1000 companies across the globe. We are consistently growing in the field of market research with more than 1000 reports published every year. The dedicated team of 400-plus analysts and consultants is committed to achieving the utmost level of our client’s satisfaction.
Contact:
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
Sales Team: sales@factmr.com
S. N. Jha
Fact.MR
+1 628-251-1583
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.